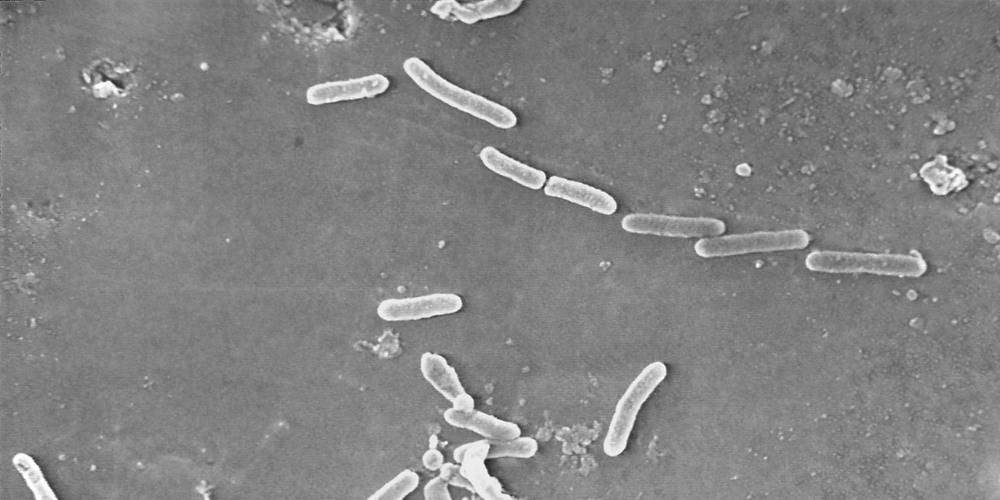
In the United States, there have been reports of people using artificial tears to infect Pseudomonas aeruginosa. Two related products, EzriCare and Delsam Pharma, were withdrawn from shelves in February. The number of infections remained at 68 in 16 states, but the death toll rose from one to three. The disease management unit continues to track the infection cases, and appeals to the public who use the artificial tears produced by the above two companies to pay attention to whether there are symptoms such as yellow or green liquid or abnormal secretions in the eyes.
According to statistics from the US Centers for Disease Control and Prevention (CDC) on the 21st, a total of 68 cases in 16 states of the United States were infected with Pseudomonas aeruginosa due to the use of artificial tears produced by the above two companies. There were 3 deaths, 8 people were blind, and 4 people were injured. Surgery to remove the eyeball. The statistics for February were 1 dead and 5 blind.
This wave of infections is particularly worrying because the strain of Pseudomonas aeruginosa infected in the individual cases is resistant to common antibiotics. The US CDC stated that this strain has never been reported in the US before.
The cases confirmed by the CDC in the United States are located in 16 states including California, New York, New Jersey, Connecticut, Illinois, Texas, and Pennsylvania, but they did not mention where the serious cases are located. At least 37 cases belong to 4 regions Groups, the artificial tears used in these 4 cases are only EzriCare products.
The CDC pointed out that more than 10 brands of artificial tears were used in the infected cases, and some patients used multiple brands, but the most infected people used EzriCare artificial tears. The CDC warns the public that they should stop using EzriCare artificial tears immediately.
CDC reminds people who have used artificial tears produced by EzriCare and Delsam Pharma to pay attention to whether there are signs of eye infection, such as: yellow, green or colorless discharge in the eyes, pain and discomfort in the eyes, red eyes or eyelids, foreign body sensation in the eyes , Increased photophobia in the eyes, blurred vision, etc. Those who have the above situation should seek medical treatment as soon as possible.
The recalled artificial tear products are produced by Global Pharma Healthcare, an India-based company. Pseudomonas aeruginosa infection outbreaks usually occur in hospitals, and the bacteria can be transmitted through contaminated hands or medical equipment.
The CDC did not disclose the patient’s personal information, but last week, an elderly woman in Florida filed a lawsuit against EzriCare on behalf of her lawyer. “JAMA Ophthalmology” (JAMA Ophthalmology) also mentioned 2 cases on the 22nd.
CNN reported that a 72-year-old woman who used EzriCare artificial tears was blind in her left eye. The woman didn’t realize that something was wrong until her vision was blurred for several days and yellow secretions were found in the pillow. She went to the emergency room and found the cornea of her left eye Large ulcers covering almost the entire eye, neither treatment nor surgery can save vision.
The other person is a 72-year-old man. Except for dry eyes, he has no other abnormalities. He also used EzriCare artificial tears. Afterwards, his eyes suffered from severe eye pain all day long and his vision decreased. Antibiotics did not work. He has already fallen into corneal blindness. It’s possible to get better.
Article source: Deaths from contaminated eyedrops rise to 3 — with 4 people needing eyeballs removed
careful! Death-threatening artificial tears
have caused 3 deaths in the United States