New drugs to treat debilitating menopause symptoms — namely hot flashes — are finally on the market or are in development. But doctors say insurance companies either won’t pay for them or make women try and fail with other, perhaps less effective, drugs before they do.
The conundrum has left women who might benefit from the new drugs at the mercy of health insurance companies.
“It’s not like a doctor can write a prescription and you go to the pharmacy and pick it up,” said Alina Salganicoff, senior vice president and director of women’s health policy at KFF, a nonprofit health policy research organization. “New drugs tend to cost a lot of money, and insurance plans can be very reluctant to cover them.”
Hormone replacement therapy is often recommended during menopause, but some women, such as breast cancer patients, can’t take it because the added hormones could fuel their cancer.
Newer drugs, however, have nothing to do with hormones.
On Thursday, Bayer announced that its nonhormonal drug, elinzanetant, “significantly” reduced the number and severity of hot flashes — intense bursts of body heat that can occur day or night — among women in clinical trials. Elinzanetant works by targeting two receptors in the brain. One, called NK-3, regulates body temperature. The other, NK-1, also affects mood and sleep.
Indeed, participants said they slept better during the time they were on the pill in the trial.
Dr. JoAnn Pinkerton, a professor of obstetrics and gynecology and the director of the Midlife Health Center at the University of Virginia School of Medicine, led one of the elinzanetant trials.
“We haven’t seen the full data,” she said, but “it did work very well to reduce frequency and severity of hot flashes. It improved sleep, and it improved overall quality of life.”
Bayer said it plans to submit its data to the Food and Drug Administration this year. If it’s approved, it would be the second nonhormonal drug for hot flashes.
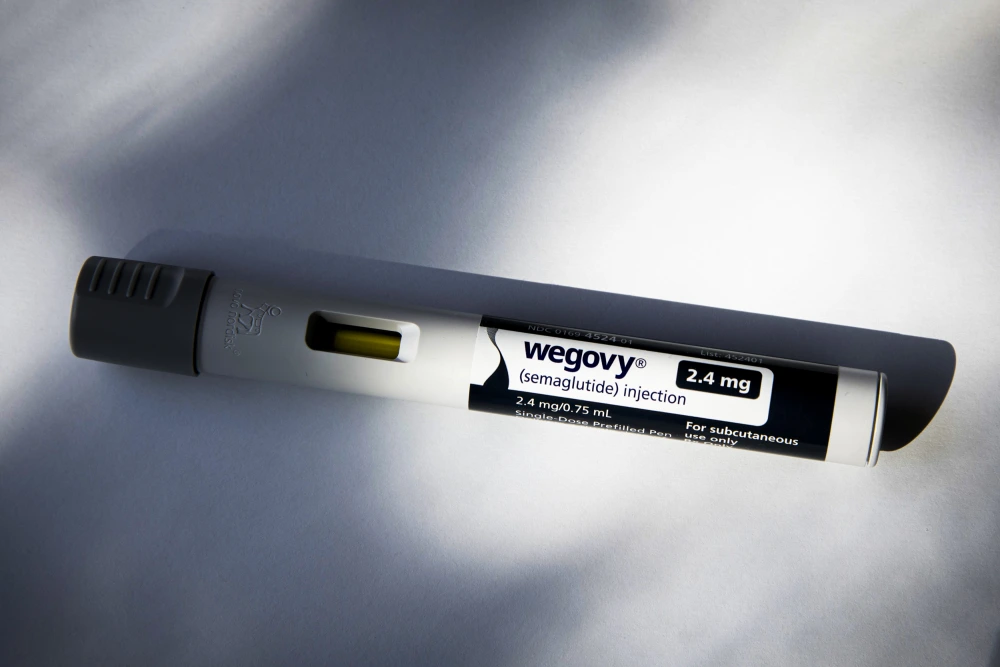
Last year, the FDA greenlighted the Astellas drug fezolinetant, sold as Veozah. It targets NK-3, the receptor that controls body temperature. Ongoing research recently found that it can reduce hot flashes for nearly six months.
Celebrities have begun to speak publicly about the condition, which once seemed taboo, even though it’s experienced by about half of the population.
Earlier this month, for example, actor Halle Berry screamed, “I’m in menopause!” at a media event on Capitol Hill to help unveil a congressional bill to boost menopause care.
“Menopause is having a moment,” said Dr. Rajita Patil, director of the Comprehensive Menopause Care Program at UCLA Health in California. “But it really should have happened well before this. It’s not like menopause is some new diagnosis.”
Still, some insurance companies are reluctant to pay for newer menopause therapies. Kaiser Permanente, for example, requires women to try at least two other drugs that haven’t been approved by the FDA to treat hot flashes before they switch to Veozah (fezolinetant), which can cost at least $550 for a month’s supply. Those drugs can include off-label use of the pain medication gabapentin or an antidepressant sold as Effexor.
“Women are being asked to try drugs that are not specifically FDA-approved for management of hot flashes before they get to a drug that actually is FDA-approved to treat hot flashes,” said Dr. Stephanie Faubion, medical director of the North American Menopause Society and director of the Mayo Clinic Center for Women’s Health. “It’s frustrating.”
Pinkerton said, “I think that most providers feel hampered in their ability to just decide what the best medication is for their patients.”
Bayer wouldn’t disclose elinzanetant’s price until the FDA clears it. It could cost as much as or more than Veozah, analysts suggest.
Cindy Laughery, 60, a pediatric organ transplant nurse at the University of Virginia School of Medicine, started having hot flashes and trouble sleeping — hallmark signs of menopause — about six years ago.
“I would turn really red and perspire really bad,” Laughery said. “People would stop me and ask if I was OK.”
She felt those sudden bursts of heat at least 10 to 15 times a day, she said. It took hours for her to fall asleep at night. She signed up for Bayer’s elinzanetant trial.
It was a “life changer,” Laughery said. Her hot flashes became rare. When they did occur, they were much milder.
“It was a revelation,” she said. “All of a sudden feeling like ‘wow, I can get up and I feel really good.’”
While Laughery said she didn’t feel any negative side effects, Pinkerton said the drug was associated with fatigue, headache and muscle aches in some study participants.
Salganicoff of KFF offers these tips:
Go to your insurance company’s website and search for your medication. The site should tell you the exact steps to take for drug coverage.
Contact your doctor’s office. Physicians often receive coupons or have insider knowledge about drug discounts. They can also act as patient advocates.
Flex your patient rights and appeal insurance denials. “Just because the first answer is ‘no’ doesn’t mean that that’s the final answer,” Salganicoff said. “This is where persistence pays off.”
nbcnews
New menopause drugs treat hot flashes, but women may face insurance hurdles