WASHINGTON — The federal government has burned through more than $1 billion to study long Covid, an effort to help the millions of Americans who experience brain fog, fatigue, and other symptoms after recovering from a coronavirus infection.
There’s basically nothing to show for it.
The National Institutes of Health hasn’t signed up a single patient to test any potential treatments — despite a clear mandate from Congress to study them. And the few trials it is planning have already drawn a firestorm of criticism, especially one intervention that experts and advocates say may actually make some patients’ long Covid symptoms worse.
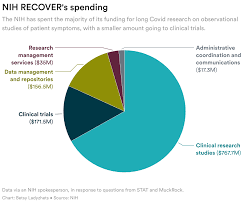
Instead, the NIH spent the majority of its money on broader, observational research that won’t directly bring relief to patients. But it still hasn’t published any findings from the patients who joined that study, almost two years after it started.
There’s no sense of urgency to do more or to speed things up, either. The agency isn’t asking Congress for any more funding for long Covid research, and STAT and MuckRock obtained documents showing the NIH refuses to use its own money to change course.
“So far, I don’t think we’ve gotten anything for a billion dollars,” said Ezekiel Emanuel, a physician, vice provost for global initiatives, and co-director of the Healthcare Transformation Institute at the University of Pennsylvania. “That is just unacceptable, and it’s a serious dysfunction.”
Eric Topol, the founder and director of the Scripps Research Translational Institute, said he expected the NIH would have launched many large-scale trials by now, and that testing treatments should have been an urgent priority when Congress first gave the agency money in late 2020.
“I don’t know that they’ve contributed anything except more confusion,” Topol said.
Patients and researchers have already raised alarms about the glacial pace of the NIH’s early long Covid efforts. But a new investigation from STAT and MuckRock, based on interviews with nearly two dozen government officials, experts, patients, and advocates, and internal NIH correspondence, letters, and public documents, underscores that the NIH hasn’t picked up the pace — instead, the delays have compounded.
It’s difficult to pinpoint exactly why progress is so stalled, experts and patients involved in the project emphasized, because the NIH has obscured both who is in charge of the long Covid efforts and how it spent the money. The broader Biden administration has also missed opportunities for oversight and accountability of the effort — despite the president’s lofty promises to focus on the disease.
The NIH’s blunders have massive ramifications for the more than 16 million Americans suffering from long Covid, in addition to those with other, similar chronic diseases. As the biggest government-funded study on this topic, the NIH initiative, dubbed RECOVER, sets precedents for future research and clinical guidelines. It will dictate how doctors across the country treat their patients — and, in turn, impact people’s ability to access work accommodations, disability benefits, and more.
“The NIH RECOVER study is pointless,” said Jenn Cole, a long Covid patient based in Brooklyn, N.Y., who wanted to enroll in the study but found the process inaccessible. The research is “a waste of time and resources,” she said, and fails to use patients’ tax dollars for their benefit.
In response to STAT and MuckRock’s questions, the NIH and an institute at Duke University managing the clinical trials defended the initiative, without providing a clear explanation for the delays.
The NIH said it chose to fund a large-scale research program instead of small-scale studies to make sure data and processes could be shared across different groups of patients, adding that clinical trials will be launching soon. In these trials, standardized study designs will allow the agency to test multiple treatments across multiple sites. If there are signals a drug works, the agency said it can pivot to devote more resources there.
A Department of Health and Human Services spokesperson said the agency has made progress over the last year in responding to long Covid, and that there are research efforts underway in addition to the RECOVER program.
“The Administration remains committed to addressing the longer-term impacts of the worst public health crisis in a century,” HHS said.
In 2020, Congress made an investment of $1.2 billion to learn more about the mysterious ongoing symptoms that were afflicting some people infected with Covid-19. That sort of money to fund research into a chronic condition like long Covid was virtually unheard of.
The money was explicitly earmarked to fund both research to understand the disease and clinical trials to test treatments that could bring patients relief. But more than two years in, the agency hasn’t started testing a single treatment. Nor is it planning to test many in the future. Instead, it’s focused on observational research — and that, too, has produced few insights.
The NIH is planning five clinical trials, each of which will test treatments that may help with a major category of long Covid symptoms. Some of these treatments will be drugs, while others will be behavioral therapies, such as cognitive retraining. Each trial will include 300 to 900 patients, selected based on their symptoms, according to details shared during a webinar in mid-April.
The only trial to be formally announced so far will focus on Paxlovid, testing whether the drug alleviates symptoms by mitigating any ongoing viral infection in patients’ bodies. The study was supposed to start recruiting in January.
But as of April, RECOVER hasn’t signed up a single patient for any of those clinical trials. And the timeline has slipped over and over again.
Initially, in a letter to members of Congress prompted by STAT’s March 2022 reporting on the initiative’s slow start, the NIH told lawmakers that the agency expected to launch clinical trials by that fall. But by August, the estimated launch had slipped to “by the end of 2022.” Then, another delay became public in December, when one of the NIH officials leading RECOVER told advisers that clinical trials would begin by the first quarter of 2023. Now, Duke University, which is overseeing the clinical trial infrastructure, told STAT and MuckRock it expects the first patients to sign up for trials this summer.
Emanuel said the pace of trials shows little urgency on the part of NIH.
“If you don’t have the pathobiology figured out, you try things. You don’t just slow, slow, slow, walk it,” he said.
All five clinical trial protocols are going through safety reviews, and the Food and Drug Administration is reviewing the trials that will test Paxlovid and other drugs, the Duke Clinical Research Institute said. The institute plans to share these protocols publicly when reviews are complete, but did not provide an estimate for when that will happen.